Abstract
Introduction: Chimeric antigen receptor T-cell (CAR-T) therapy is an effective treatment that has transformed hematologic malignancy management. However, its significant short-term toxicities, mainly cytokine release syndrome (CRS) and neurotoxicity, require frequent monitoring almost universally achieved via prolonged inpatient admissions. We have reported our institution's specialized, multi-disciplinary hospital-based outpatient (HBO) CAR-T service and experience (Bansal et.al, ASCO 2021). To enhance the HBO practice, we implemented a remote patient monitoring (RPM) program, comprised of in-home, electronic health record-integrated technology to monitor vital signs and neurologic symptoms for 30 days post CAR-T infusion. The RPM program's triaging algorithm generates alerts based on predetermined parameters to guide escalation of care if needed. In this report, we describe the characteristics of RPM program patients (pts), focusing on the alerts received within 48 hours (h) of first hospitalization in the post-CAR-T setting.
Methods: Pts undergoing commercial CAR-T cell therapy at Mayo Clinic Rochester were enrolled in the RPM program as part of our standard of care from 06/2020 to 05/2021. Pts with detailed RPM data available were included in this analysis. Patient characteristics, health care utilization, inpatient interventions, and RPM alert data were reviewed. Wilcoxon test was used for continuous variables, chi-squared test was used for categorical variables.
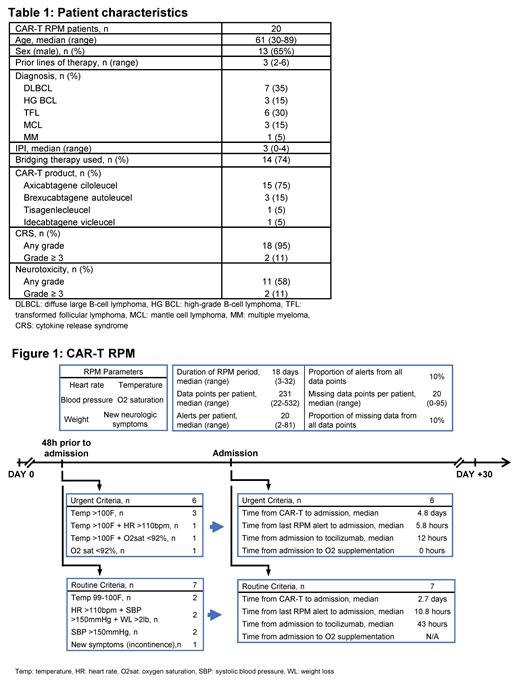
Results: Baseline characteristics are summarized in Table 1. Of 20 pts in this analysis, 13 had RPM alerts in the 48h preceding the first hospitalization post CAR-T. For the remaining 7 pts without RPM alerts, 3 were hospitalized within 24h of CAR-T infusion without significant RPM data, 1 patient hospitalized with acute pancreatitis (day +8 post CAR-T infusion), 1 for doubling of CRP (day +10), 1 for acute pain crisis (day +5), and 1 with neutropenic fever (day +5) with not reporting RPM data.
The median RPM program duration was 18 days (range 3-32) which accounts for interruptions in the RPM monitoring while pts are hospitalized. The median number of data points per patient was 231 (range 22-532), with a median of 20 alerts per patient (range 2-81). The median missing data points per patient were 20 (10%) (range 0-95).
Of the 13 pts with RPM alerts within 48h of first hospitalization, alerts met our definition of urgent criteria in 6 pts [temperature (temp) > 100F (n=3), temp > 100F + tachycardia (n=1), temp > 100F + hypoxia (n=1), and hypoxia (n=1)]. In the remaining 7 pts, the RPM alerts met our routine criteria definition [temp 99-100 (n=2), tachycardia + hypertension + weight loss (n=2), hypertension (n=2), new incontinence (n=1)]. CRS was reported in 12 pts [grade 1-2 (n=11), grade 3-4 (n=1)] and neurotoxicity in 8 pts [grade 1-2 (n=7), grade 3-4 (n=1)]. The median post CAR-T first hospitalization day was day +4 (range day +2-10), Figure 1.
The median time from the last RPM alert to hospitalization was 5.8h for pts meeting urgent criteria vs 10.8h for those meeting routine criteria (p=0.13). Tocilizumab was used in 5 pts during the first hospitalization [3 (50%) in the urgent criteria group and 2 (28%) in the routine criteria group], with a median time from admission to tocilizumab of 12h in the urgent criteria group vs 43h in the routine criteria group (p=0.05). Early hospitalization (≤ 3 days) post CAR-T infusion was associated with later need for tocilizumab [median 51h (range 30-57) vs. 11.5h (range 6-33) from admission, p=0.03] than late hospitalization (> 4 days post CAR-T). Upon admission, oxygen supplementation was started on both pts with hypoxia noted in the RPM alerts. Only 1 patient (routine criteria group) required use of vasopressors while hospitalized, initiated more than 48h after admission.
Conclusion: A CAR-T RPM program can identify adverse symptoms and vital sign changes in CAR-T pts managed in the outpatient setting. In this pilot implementation, the CAR-T RPM triaging algorithm identified pts requiring expedited hospitalization and facilitated early initiation of tocilizumab. The intermittent monitoring and proportion of missing data are the main limitations of the RPM in the acute care setting post-CAR-T infusion. Incorporation of wearable devices for continuous remote monitoring is being explored as a mitigating strategy to these limitations.
Paludo: Karyopharm: Research Funding. Dingli: Apellis: Consultancy; Janssen: Consultancy; GSK: Consultancy; Novartis: Research Funding; Sanofi: Consultancy; Alexion: Consultancy. Kapoor: Glaxo SmithKline: Research Funding; Sanofi: Consultancy; Amgen: Research Funding; AbbVie: Research Funding; Sanofi: Research Funding; Karyopharm: Research Funding; Ichnos Sciences: Research Funding; Pharmacyclics: Consultancy; Takeda: Research Funding; Regeneron Pharmaceuticals: Research Funding; Karyopharm: Consultancy; Cellectar: Consultancy; BeiGene: Consultancy. Gertz: Aurora Biopharma: Other: Stock option; Ionis Pharmaceuticals: Other: Advisory Board; Akcea Therapeutics, Alnylam Pharmaceuticals Inc, Prothena: Consultancy; AbbVie Inc, Celgene Corporation: Other: Data Safetly & Monitoring; Akcea Therapeutics, Ambry Genetics, Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Karyopharm Therapeutics, Pfizer Inc (to Institution), Sanofi Genzyme: Honoraria. Wang: InnoCare: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Genentech: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kenderian: Humanigen, Inc.: Consultancy, Honoraria, Research Funding. Kumar: Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Oncopeptides: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche-Genentech: Consultancy, Research Funding; Carsgen: Research Funding; Antengene: Consultancy, Honoraria; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Bluebird Bio: Consultancy; Beigene: Consultancy; Tenebio: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding. Bennani: Purdue Pharma: Other: Advisory Board; Daichii Sankyo Inc: Other: Advisory Board; Kyowa Kirin: Other: Advisory Board; Vividion: Other: Advisory Board; Kymera: Other: Advisory Board; Verastem: Other: Advisory Board. Ansell: Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium and Takeda: Research Funding. Lin: Merck: Research Funding; Vineti: Consultancy; Gamida Cell: Consultancy; Juno: Consultancy; Bluebird Bio: Consultancy, Research Funding; Legend: Consultancy; Celgene: Consultancy, Research Funding; Sorrento: Consultancy; Takeda: Research Funding; Janssen: Consultancy, Research Funding; Novartis: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal